Search
Found 56 results

Our Mission
OICR Genomics OICR Genomics is an OICR/UHN collaborative initiative led by Dr. Trevor Pugh that supports basic, translational, and clinical oncogenomics projects. We offer CAP/ACD-accredited, CLIA-certified, ISO 15189-compliant services for your clinical reporting and research needs. For over 10 years, our teams have supported research across Canada and around the world by solving genomics and […]
Our Methylome Sequencing Services
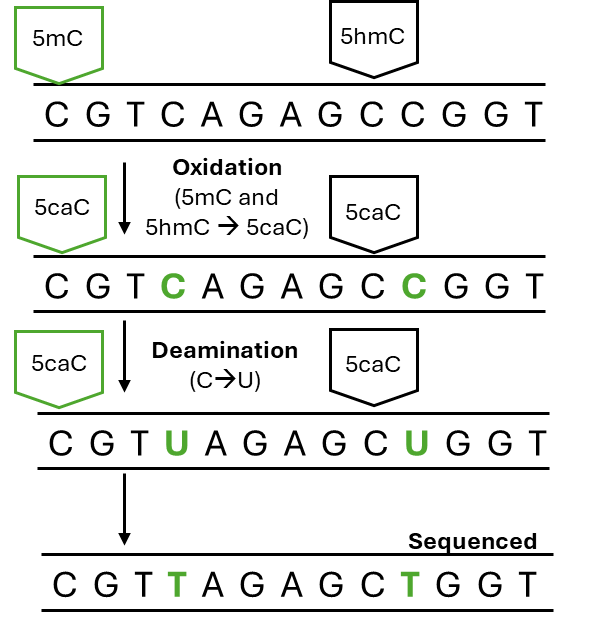
Enzymatic Methyl-seq (EM-seq) EM-Seq relies on the NEBNext Enzymatic Methyl-seq kit to enzymatically convert unmethylated cytosines to uracil, preserving the methylated cytosine as cytosine. During PCR, uracil gets converted to thyme. As a result, unmethylated cytosines are sequenced as thyme and methylated cytosines are sequenced as cytosine. This assays allows for the identification of methylated regions at […]

Sample Requirements and Sequencing Information
Whole Genome and Transcriptome Sequencing WGTSClinical and Research (RUO) WGSClinical and Research (RUO) WTSResearch Only (RUO) pWGSClinical and Research (RUO) Sample Requirements DNA230 ng (gDNA from buffy coat)80 ng (gDNA from fresh frozen)130 ng (gDNA from FFPE) RNA80 ng (RNA from fresh frozen)275 ng (RNA from FFPE) 230 ng (gDNA from buffy coat)80 ng […]

Glossary
Assays Whole Genome and Transcriptome Sequencing Plasma Whole Genome Sequencing Targeted Sequencing Whole Exome Sequencing Methylome Sequencing Library Preparation Protocols Whole Genome Library Preparation Whole Genome Library Preparation with Hamilton STAR Liquid Handler Robot Whole Transcriptome Library Preparation Plasma Whole Genome Library Preparation Plasma Whole Genome Library Preparation with Sciclone G3 Liquid Handler Targeted Sequencing […]

Methylome Sequencing
Our Project Workflow Resources Sample Requirements and Sequencing Information Library Preparation Protocols Bioinformatics Analysis Pipeline (COMING SOON!) Learn more about our methylome sequencing assays Why work with us? CAP/ACD-accredited, CLIA-certified, ISO 15189-compliant facility Robust quality control protocols Automated informatics pipeline Continuous support from our scientists and staff We would love to connect with you and […]

Targeted Sequencing
Resources Sample Requirements and Sequencing Information Library Preparation Protocols Bioinformatics Analysis Pipeline (COMING SOON!) Clinical Report Deliverable Example Our Project Workflow Why work with us? CAP/ACD-accredited, CLIA-certified, ISO 15189-compliant facility Robust quality control protocols Automated informatics pipeline Continuous support from our scientists and staff We would love to connect with you and learn more about […]

Whole Genome and Transcriptome Sequencing
Our Project Workflow Resources Sample Requirements and Sequencing Information Library Preparation Protocols Bioinformatics Analysis Pipeline (COMING SOON!) Clinical Report Deliverable Example Why work with us? CAP/ACD-accredited, CLIA-certified, ISO 15189-compliant facility Robust quality control protocols Automated informatics pipeline Continuous support from our scientists and staff We would love to connect with you and learn more about […]

Ultima Sequencing
Applications Bioinformatics Support from Ultima is now available! More information can be found here! Pricing Service List Price (CAD) Minimum Order Wafer (10B) $5130 2 Wafers 30X WGS $435 20 Samples 30X EM-Seq $485 20 Samples WGS (80T/30N) $2050 6 Cases 200X cfDNA WGS $1700 6 Samples ppm-seq (100X raw, ~30X high quality ppm-Seq data) […]

Liquid Biopsy – OMPRN
Learn about a relatively new medical testing technique called liquid biopsy, courtesy of Ontario Molecular Pathology Research Network.